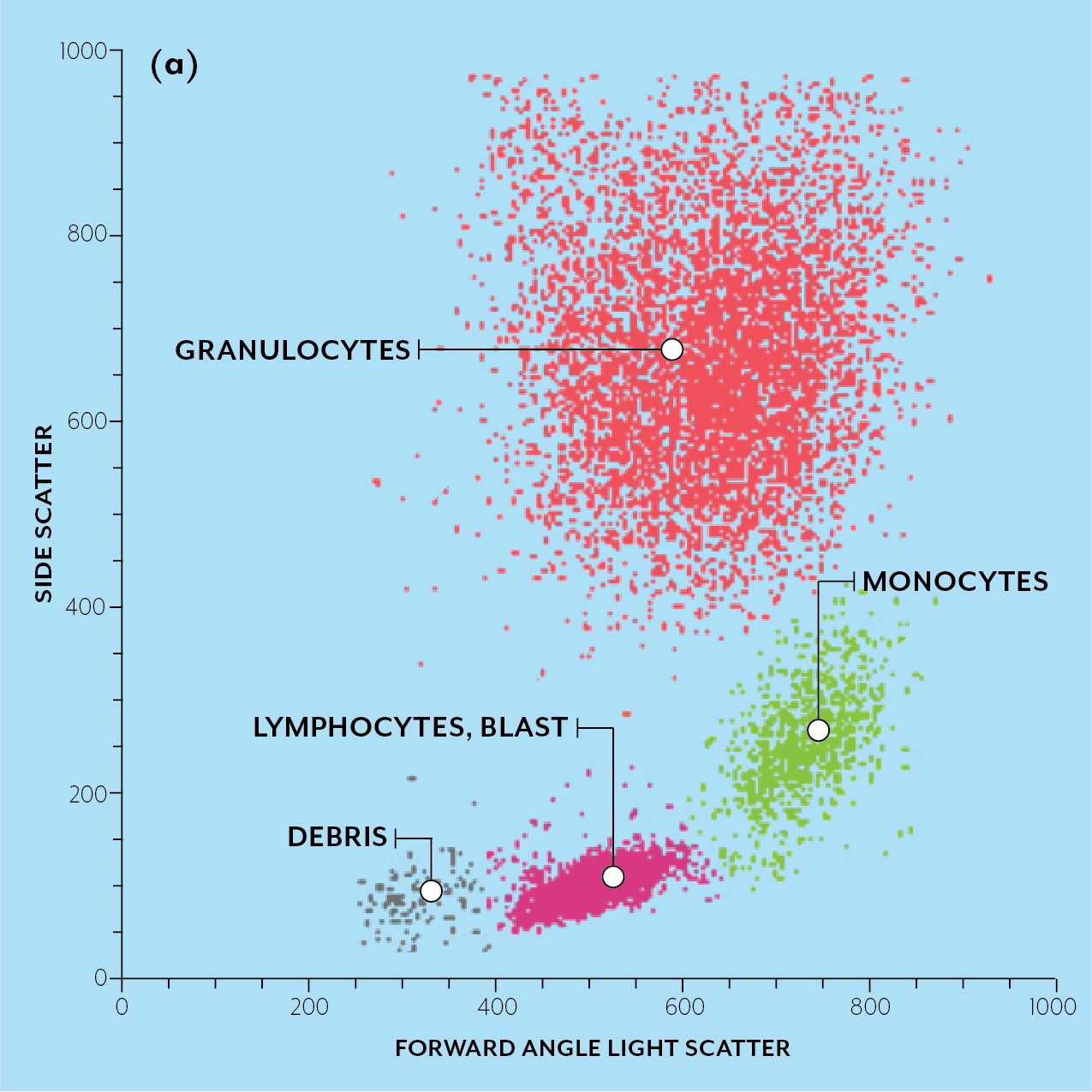
Flow cytometry is a method to analyze individual cells as they flow past a detector. For a good overview of the technique, see this review article or this PDF. There are 3 main types of flow cytometry:
1. Cell counting, uses optical and impedance methods to rapidly count and characterize cells, this is the fastest method.
- Applications include:
- Process monitoring for cell-culture-based processing methods
- Environmental, to characterize algae populations
- Clinical, blood banks and hematology labs
2. Cell sorting, uses optical and impedance methods to sort cells into populations.
- Used to separate cells of a certain type for further use
- scDNA sequencing
- PCR, etc.
3. Imaging flow cytometry, cells are imaged as they pass through the system.
- Typically used in conjunction with cell sorting for
- Biological research
- Immunology research
Omega provides custom filters for your Flow Cytometry system
- Custom angle-of-incidence (AOI) requirements (read our tech note about AOI effects)
- Custom transmission wavelengths
- Custom blocking requirements
- Custom sizes
- Quantities up to thousands per year
Filters for Multiplexing in Flow Cytometry
The ability to detect many target molecules or cellular properties (such as scattering coefficient) nearly simultaneously is termed "multiplexing". Proper multiplexing requires excellent separation between interfering signals, which means optimizing the geometry and filters in the system. Flow cytometers employ a combination of light scattering and fluorescence to optically sort and characterize cells. The most complicated systems use 7 lasers at once. Each laser is associated with a detection module that splits the signal into the scattering and fluorescence channels. Up to 5 fluorescence signals can be detected using a single laser excitation wavelength.
Factors to consider in the design phase include:
-
Number of fluorescence signals excited and detected at once and their relative intensities:
Companies have designed series of dyes to sample the entire visible and NIR spectrum using excitation at a single wavelength.
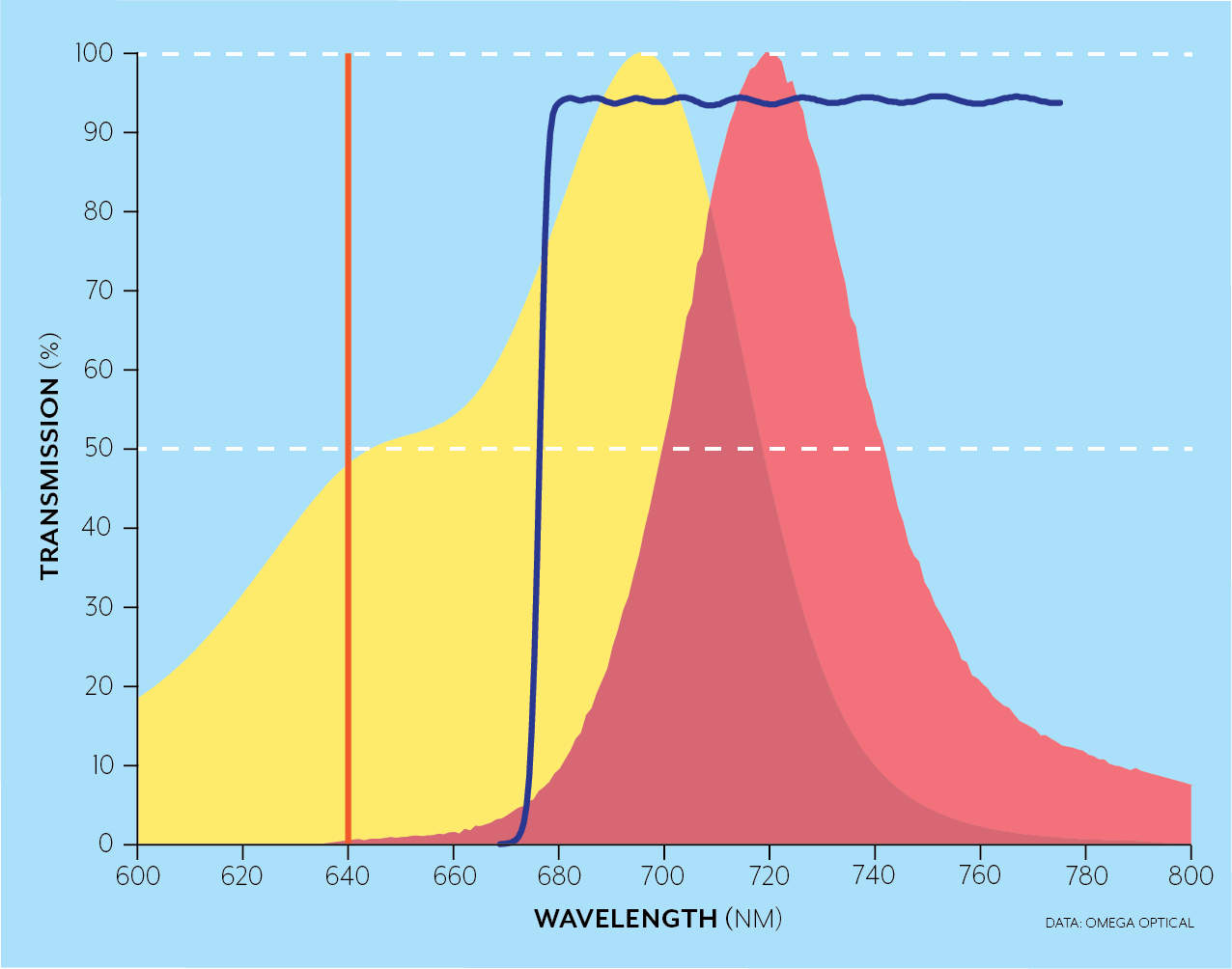
For instance, the Brilliant Violet dyes were developed to emit in wavelength bands over the full spectral range while being excited simultaneously by the 405nm laser. In the examples below, for a 640 nm laser excitation (orange), a longpass filter (blue line) can be used when a single fluorophore like AlexaFluor 700 (yellow and red areas) is excited as in the top figure, while dichroics (not pictured) and bandpass filters (crimson and blue) can be used to separate the emission from several fluorophores (AlexaFluor 647 in blue/green and AlexaFluor 700 yellow/red in this case) in the bottom figure.
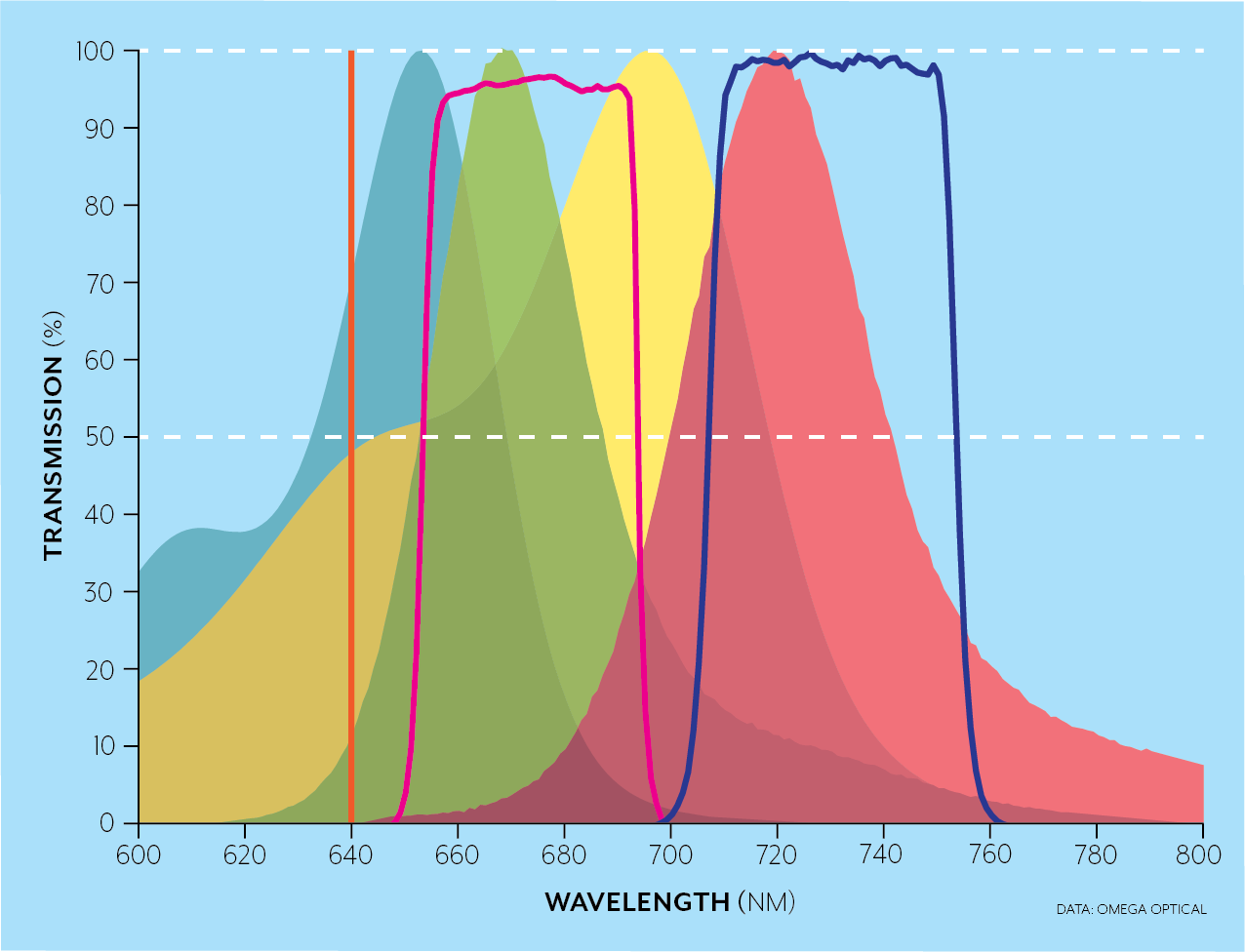
-
Geometry of the dichroics and detectors in flow cytometry
- Dichroics used at low angles (below about 10 deg) exhibit the steepest edges between transmission and reflection- our AOI tech note explains why
- Very high transmission and reflection values in a long train of dichroics and detector to reduce losses through the system (>95% minimum)
- Wavefront distortions of the dichroics (transmitted and/or reflected), especially in a long detection train can cause signal loss if the beam is being deflected or expanding through the optical train
- Dichroic mounting procedures can induce wavefront distortions and beam steering issues if not considered early in the process
-
Throughput and wavefront distortion can often be calibrated out of the system, but proper specifications at the design phase can eliminate problems later
- Emission filters are typically placed directly in front of the detector
- Flatness and wavefront are less important
- Blocking of scattering and other fluorescence signals is important
- Blocking of dichroics and emission filters work together to provide spectral separation
-
Blocking of extended wavelength regions increases complexity and cost of the filters- work with us to maximize performance and minimize cost
Flow Cytometry Multiplexing with Spectral Detection
Another method, called spectral flow-cytometry, uses a dispersing element (grating or prism) to produce a full-spectrum instead of using individual wavelength bands of interest.
The spectra are then "unmixed" to provide details about the different signals in the sample.

Omega's partner, Optometrics, manufactures ruled, holographic and replicated gratings for use in spectral flow cytometers and spectrometers.
Omega produces linear variable longpass filters and patterned order-sorting filters to use with these gratings to eliminate higher orders from reaching the detector and producing signal artifacts.
