Flow cytometers employ electrical (Coulter detection), light scattering and fluorescence to count, sort and characterize cells. Optical methods employ a laser beams and sometimes LED light beams which the cells pass through one at a time.
Light scattering in both the forward and side (perpendicular) directions indicate whether a cell or particle is present in the beam. Forward scattering strength is proportional to the size of the cell while side scattering is related to the intracellular composition of the cell. A scatterplot of forward to side-scattering signals can be used to sort or count certain cell types (leukocytes versus monocytes for instance) Light is scattered at the same wavelength as the laser. Instruments used in hematology labs use this and the Coulter method to count cells of various types.
Fluorescence detection is also commonly used in the perpendicular (side-scatter) direction. Some cells (i.e. algae) have intrinsic fluorescence that can be used for sorting, but most cells require fluorescent labels to impart fluorescence to specific regions inside the cell. Typically these fluorescent labels bind to specific proteins of interest within the cells.
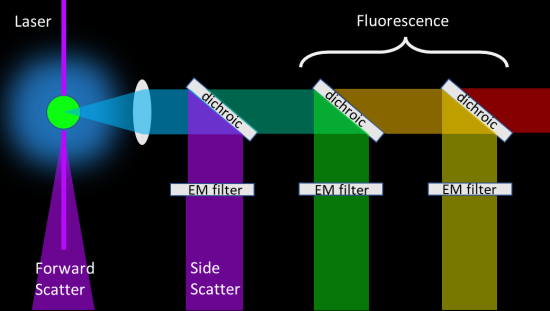
The forward scatter signal is quite strong and has a block so the laser won't directly enter the detector. For the side scatter channel, filters are required to separate the side scatter from the fluorescence signals. A typical detector module contains the following:
1. Side-scatter channel containing
- long-pass dichroic that reflects the scattered laser light into the detector and transmits the fluorescence signals
- a narrow-band laser filter in front of the detector
2. A series of fluorescence channels consisting of
- a series of long-pass dichroic filters
- a complementary series of bandpass filters in front of a series of detectors
- the filter wavelengths are selected to match the fluorescent dyes used in the system
- 5 (or more) fluorescent labels can be excited by a single laser wavelength
Note that there are many geometries for the detector modules, but dichroics and emission filters play a central role. The number of detection modules is equal to the number of lasers in the system. The most complicated systems can have upwards of 7 lasers! This means the cells can be characterized with 35 or more fluorescent labels and 14 scatter channels! This degree of multiplexing makes analysis very complicated.
